- The partnership will support the progression of PY314, the first of Pionyr’s Myeloid TuningTM antibodies to enter the clinic
- Pionyr will use Abcam’s EPR20243 clone to evaluate TREM2 levels in tissue samples for potential future development as a CDx
- Abcam is Pionyr’s preferred partner for the long-term provision of anti-TREM2 antibody, suitable for use throughout the diagnostics development process
South San Francisco, US and Cambridge, UK – 22 June 2021: Today Pionyr Immunotherapeutics, Inc., a company developing first-in-class antibody therapeutics that increase the body’s anti-tumor immunity and Abcam (AIM:ABC; NASDAQ:ABCM), a global innovator in life science reagents and tools, announced the extension of their collaboration with a new commercial licensing agreement to support the progression of PY314, the first to the clinic of Pionyr’s compounds.
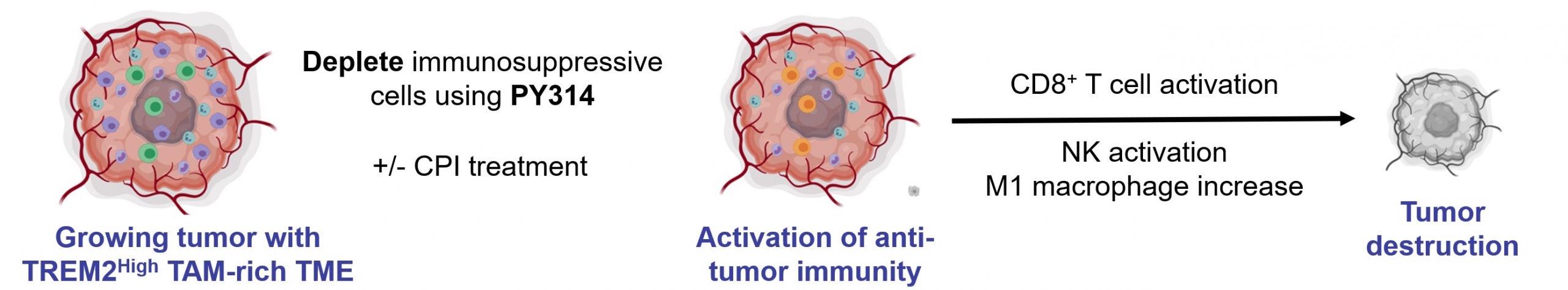
Currently in Phase 1 clinical study(1), PY314 is an anti-TREM2 (Triggering Receptor Expressed on Myeloid cells 2) monoclonal antibody for the treatment of advanced solid tumors. PY314 is designed to tune the tumor microenvironment by selectively depleting immune suppressive TREM2-expressing tumor-associated macrophages to promote anti-tumor immunity.
..
“We are excited to collaborate with Abcam to support the clinical development of our PY314 program for the treatment of solid tumors. Abcam’s in-depth knowledge and track record of successfully supporting diagnostic assay development is invaluable to anticipate late-stage development requirements, and to facilitate access to the right expertise at the right time”
Kevin Baker
SVP and Chief Development Officer at Pionyr
“We are excited to collaborate with Abcam to support the clinical development of our PY314 program for the treatment of solid tumors. Abcam’s in-depth knowledge and track record of successfully supporting diagnostic assay development is invaluable to anticipate late-stage development requirements, and to facilitate access to the right expertise at the right time”
Kevin Baker
SVP and Chief Development Officer at Pionyr
Pionyr’s Phase 1 clinical study will recruit patients with predefined tumor types where macrophages expressing TREM2 in the tumor microenvironment are most likely implicated as a driver of resistant metastatic disease. Under the terms of the agreement, Pionyr will evaluate Abcam’s anti-TREM2 antibody to detect the presence of TREM2-expressing macrophages in tumor biopsy samples from patients enrolled in the first-in-human study. Pionyr will explore the potential for developing a companion diagnostic test leveraging Abcam’s high quality reagents and end-to end-expertise to facilitate and de-risk the diagnostics development journey.
“Pionyr’s Phase 1 PY314 is a promising therapy that has the potential to unlock durable benefits for cancer patients. Precise monitoring of the presence of TREM2 in tumor biopsy samples throughout the clinical development process, enabled by Pionyr’s use of Abcam’s EPR20243 clone, will provide vital support to the success of the trial. We are thrilled to be working with Pionyr and supporting their efforts to enhance the immune system’s anti-tumor response”
John Baker
SVP Business Development at Abcam
“Pionyr’s Phase 1 PY314 is a promising therapy that has the potential to unlock durable benefits for cancer patients. Precise monitoring of the presence of TREM2 in tumor biopsy samples throughout the clinical development process, enabled by Pionyr’s use of Abcam’s EPR20243 clone, will provide vital support to the success of the trial. We are thrilled to be working with Pionyr and supporting their efforts to enhance the immune system’s anti-tumor response”
John Baker
SVP Business Development at Abcam
(1) Study of PY314 in Subjects With Advanced Solid Tumors – NCT04691375